How To Find Protons Electrons And Neutrons Of An Isotope
Overview of Atomic Structure
Atoms are made up of particles chosen protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.
Learning Objectives
Discuss the electronic and structural properties of an atom
Central Takeaways
Key Points
- An atom is composed of ii regions: the nucleus, which is in the center of the cantlet and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.
- Protons and neutrons have approximately the aforementioned mass, about ane.67 × 10-24 grams, which scientists define every bit ane diminutive mass unit of measurement (amu) or one Dalton.
- Each electron has a negative accuse (-i) equal to the positive charge of a proton (+1).
- Neutrons are uncharged particles found within the nucleus.
Key Terms
- cantlet: The smallest possible amount of affair which still retains its identity as a chemical element, consisting of a nucleus surrounded past electrons.
- proton: Positively charged subatomic particle forming office of the nucleus of an atom and determining the atomic number of an element. It weighs 1 amu.
- neutron: A subatomic particle forming office of the nucleus of an atom. It has no charge. It is equal in mass to a proton or it weighs 1 amu.
An atom is the smallest unit of measurement of matter that retains all of the chemic properties of an chemical element. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. For example, water is composed of hydrogen and oxygen atoms that have combined to course h2o molecules. Many biological processes are devoted to breaking downwardly molecules into their component atoms so they can be reassembled into a more useful molecule.
Atomic Particles
Atoms consist of three basic particles: protons, electrons, and neutrons. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and incorporate the electrons (negatively charged). Atoms have unlike properties based on the arrangement and number of their basic particles.
The hydrogen cantlet (H) contains simply ane proton, one electron, and no neutrons. This can be adamant using the atomic number and the mass number of the element (see the concept on atomic numbers and mass numbers).
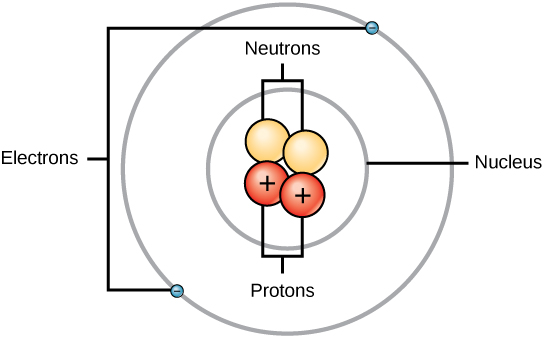
Structure of an atom: Elements, such every bit helium, depicted here, are made upward of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
Atomic Mass
Protons and neutrons take approximately the aforementioned mass, about 1.67 × ten-24 grams. Scientists define this amount of mass equally one atomic mass unit (amu) or ane Dalton. Although similar in mass, protons are positively charged, while neutrons have no accuse. Therefore, the number of neutrons in an atom contributes significantly to its mass, simply not to its accuse.
Electrons are much smaller in mass than protons, weighing just 9.xi × 10-28 grams, or near 1/1800 of an atomic mass unit of measurement. Therefore, they do not contribute much to an element'due south overall atomic mass. When considering atomic mass, it is customary to ignore the mass of any electrons and calculate the atom's mass based on the number of protons and neutrons alone.
Electrons contribute greatly to the cantlet'south charge, equally each electron has a negative charge equal to the positive charge of a proton. Scientists define these charges as "+1" and "-ane. " In an uncharged, neutral atom, the number of electrons orbiting the nucleus is equal to the number of protons within the nucleus. In these atoms, the positive and negative charges abolish each other out, leading to an atom with no net accuse.

Protons, neutrons, and electrons: Both protons and neutrons have a mass of 1 amu and are found in the nucleus. However, protons accept a charge of +i, and neutrons are uncharged. Electrons have a mass of approximately 0 amu, orbit the nucleus, and take a charge of -1.
Book of Atoms
Accounting for the sizes of protons, neutrons, and electrons, most of the volume of an cantlet—greater than 99 percent—is, in fact, empty infinite. Despite all this empty space, solid objects do non just pass through one some other. The electrons that surround all atoms are negatively charged and cause atoms to repel ane another, preventing atoms from occupying the same infinite. These intermolecular forces preclude you from falling through an object like your chair.
Atomic Number and Mass Number
The atomic number is the number of protons in an chemical element, while the mass number is the number of protons plus the number of neutrons.
Learning Objectives
Determine the relationship between the mass number of an atom, its atomic number, its atomic mass, and its number of subatomic particles
Cardinal Takeaways
Central Points
- Neutral atoms of each element contain an equal number of protons and electrons.
- The number of protons determines an element'due south atomic number and is used to distinguish one element from another.
- The number of neutrons is variable, resulting in isotopes, which are different forms of the same atom that vary only in the number of neutrons they possess.
- Together, the number of protons and the number of neutrons decide an chemical element's mass number.
- Since an element'south isotopes have slightly different mass numbers, the atomic mass is calculated by obtaining the mean of the mass numbers for its isotopes.
Key Terms
- mass number: The sum of the number of protons and the number of neutrons in an cantlet.
- diminutive number: The number of protons in an cantlet.
- atomic mass: The average mass of an atom, taking into business relationship all its naturally occurring isotopes.
Atomic Number
Neutral atoms of an chemical element comprise an equal number of protons and electrons. The number of protons determines an element's diminutive number (Z) and distinguishes 1 element from another. For example, carbon's atomic number (Z) is 6 considering it has 6 protons. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have unlike numbers of neutrons. The number of electrons can also exist different in atoms of the same element, thus producing ions (charged atoms). For instance, iron, Atomic number 26, can exist in its neutral country, or in the +2 and +3 ionic states.
Mass Number
An element'due south mass number (A) is the sum of the number of protons and the number of neutrons. The small contribution of mass from electrons is disregarded in calculating the mass number. This approximation of mass can be used to easily summate how many neutrons an element has by just subtracting the number of protons from the mass number. Protons and neutrons both counterbalance near one diminutive mass unit or amu. Isotopes of the aforementioned element volition have the same atomic number but different mass numbers.

Atomic number, chemical symbol, and mass number: Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and 13, respectively. Its average atomic mass is 12.11.
Scientists determine the atomic mass past calculating the mean of the mass numbers for its naturally-occurring isotopes. Oft, the resulting number contains a decimal. For example, the atomic mass of chlorine (Cl) is 35.45 amu because chlorine is composed of several isotopes, some (the bulk) with an diminutive mass of 35 amu (17 protons and 18 neutrons) and some with an atomic mass of 37 amu (17 protons and twenty neutrons).
Given an atomic number (Z) and mass number (A), y'all tin discover the number of protons, neutrons, and electrons in a neutral atom. For example, a lithium atom (Z=iii, A=7 amu) contains three protons (found from Z), three electrons (as the number of protons is equal to the number of electrons in an cantlet), and four neutrons (7 – 3 = 4).
Isotopes
Isotopes are various forms of an element that have the same number of protons, just a different number of neutrons.
Learning Objectives
Discuss the properties of isotopes and their use in radiometric dating
Key Takeaways
Key Points
- Isotopes are atoms of the same chemical element that comprise an identical number of protons, but a different number of neutrons.
- Despite having different numbers of neutrons, isotopes of the same element have very similar physical properties.
- Some isotopes are unstable and volition undergo radioactivity to get other elements.
- The anticipated half-life of different decaying isotopes allows scientists to engagement material based on its isotopic composition, such as with Carbon-14 dating.
Fundamental Terms
- isotope: Whatsoever of 2 or more forms of an element where the atoms have the same number of protons, only a dissimilar number of neutrons within their nuclei.
- one-half-life: The fourth dimension it takes for half of the original concentration of an isotope to decay dorsum to its more than stable form.
- radioactive isotopes: an atom with an unstable nucleus, characterized by backlog energy available that undergoes radioactive decay and creates most unremarkably gamma rays, blastoff or beta particles.
- radiocarbon dating: Determining the age of an object past comparing the ratio of the 14C concentration plant in information technology to the amount of 14C in the atmosphere.
What is an Isotope?
Isotopes are various forms of an element that have the same number of protons but a dissimilar number of neutrons. Some elements, such every bit carbon, potassium, and uranium, take multiple naturally-occurring isotopes. Isotopes are defined first past their chemical element and so by the sum of the protons and neutrons present.
- Carbon-12 (or 12C) contains six protons, half-dozen neutrons, and six electrons; therefore, it has a mass number of 12 amu (six protons and six neutrons).
- Carbon-fourteen (or xivC) contains six protons, eight neutrons, and six electrons; its atomic mass is xiv amu (6 protons and eight neutrons).
While the mass of individual isotopes is different, their physical and chemical properties remain by and large unchanged.
Isotopes do differ in their stability. Carbon-12 (12C) is the most abundant of the carbon isotopes, accounting for 98.89% of carbon on World. Carbon-14 (14C) is unstable and only occurs in trace amounts. Unstable isotopes nearly ordinarily emit blastoff particles (He2+) and electrons. Neutrons, protons, and positrons can also exist emitted and electrons can be captured to attain a more than stable atomic configuration (lower level of potential free energy ) through a procedure called radioactive disuse. The new atoms created may be in a high energy land and emit gamma rays which lowers the energy but alone does not change the atom into some other isotope. These atoms are called radioactive isotopes or radioisotopes.
Radiocarbon Dating
Carbon is normally present in the atmosphere in the form of gaseous compounds like carbon dioxide and methane. Carbon-14 (14C) is a naturally-occurring radioisotope that is created from atmospheric fourteenN (nitrogen) by the addition of a neutron and the loss of a proton, which is acquired by cosmic rays. This is a continuous process so more 14C is always being created in the atmosphere. In one case produced, the xivC oft combines with the oxygen in the atmosphere to class carbon dioxide. Carbon dioxide produced in this way diffuses in the atmosphere, is dissolved in the bounding main, and is incorporated by plants via photosynthesis. Animals consume the plants and, ultimately, the radiocarbon is distributed throughout the biosphere.
In living organisms, the relative amount of 14C in their trunk is approximately equal to the concentration of 14C in the atmosphere. When an organism dies, it is no longer ingesting 14C, so the ratio betwixt 14C and 12C will reject equally fourteenC gradually decays back to xivN. This slow process, which is called beta decay, releases energy through the emission of electrons from the nucleus or positrons.
After approximately 5,730 years, half of the starting concentration of xivC will have been converted dorsum to fourteenN. This is referred to as its half-life, or the time it takes for half of the original concentration of an isotope to decay back to its more stable grade. Because the half-life of 14C is long, it is used to engagement formerly-living objects such as erstwhile bones or woods. Comparing the ratio of the 14C concentration constitute in an object to the amount of 14C in the atmosphere, the amount of the isotope that has not yet rust-covered can exist determined. On the basis of this amount, the historic period of the cloth can exist accurately calculated, as long as the cloth is believed to be less than 50,000 years former. This technique is chosen radiocarbon dating, or carbon dating for short.

Application of carbon dating: The age of carbon-containing remains less than 50,000 years old, such as this pygmy mammoth, can be determined using carbon dating.
Other elements have isotopes with dissimilar half lives. For instance, 40K (potassium-40) has a half-life of 1.25 billion years, and 235U (uranium-235) has a half-life of about 700 million years. Scientists often use these other radioactive elements to date objects that are older than 50,000 years (the limit of carbon dating). Through the utilize of radiometric dating, scientists can written report the historic period of fossils or other remains of extinct organisms.
The Periodic Tabular array
Everything in the universe is made of ane or more than elements. The periodic table is a ways of organizing the various elements co-ordinate to similar physical and chemical properties.
Learning Objectives
Discuss the organization of the periodic table
Key Takeaways
Key Points
- All matter is made from atoms of ane or more than elements. Living creatures consist mainly of carbon, hydrogen, oxygen, and nitrogen (CHON).
- Combining elements creates compounds that may accept emergent properties.
- The periodic table is a listing of the elements co-ordinate to increasing atomic number that is further organized into columns based on similar physical and chemic properties and electron configuration.
- Every bit one moves down a column or beyond a row, there are some general trends for the backdrop of the elements.
- The periodic table continues to expand today as heavier and heavier elements are created in laboratories around the world.
Key Terms
- chemical element: Pure chemical substances consisting of just one blazon of cantlet with a defined fix of chemical and physical backdrop.
- emergent properties: Backdrop institute in compound structures that are unlike from those of the individual components and would non be predicted based on the properties of the private components.
- periodic tabular array: A tabular chart of the chemical elements co-ordinate to their atomic numbers so that elements with similar backdrop are in the same column.
Affair and Elements
Affair comprises all of the physical objects in the universe, those that take up space and take mass. All matter is composed of atoms of one or more elements, pure substances with specific chemical and physical properties. There are 98 elements that naturally occur on earth, yet living systems use a relatively small number of these. Living creatures are composed mainly of simply four elements: carbon, hydrogen, oxygen, and nitrogen (often remembered past the acronym CHON). Equally elements are bonded together they class compounds that often have new emergent properties that are different from the backdrop of the individual elements. Life is an example of an emergent holding that arises from the specific collection of molecules found in cells.

Elements of the human trunk bundled by percent of full mass: There are 25 elements believed to play an active part in human wellness. Carbon, hydrogen, oxygen, and nitrogen make up approximately 96% of the mass in a human body.
The Periodic Table
The different elements are organized and displayed in the periodic table. Devised by Russian pharmacist Dmitri Mendeleev (1834–1907) in 1869, the table groups elements that, although unique, share certain chemical properties with other elements. In the periodic table the elements are organized and displayed according to their diminutive number and are bundled in a series of rows (periods) and columns (groups) based on shared chemical and concrete properties. If you look at a periodic tabular array, you will see the groups numbered at the acme of each column from left to correct starting with 1 and ending with eighteen. In improver to providing the atomic number for each element, the periodic table too displays the chemical element'due south diminutive mass. Looking at carbon, for example, its symbol (C) and proper name appear, as well as its diminutive number of six (in the upper left-hand corner) and its atomic mass of 12.11.

The periodic table: The periodic table shows the atomic mass and atomic number of each chemical element. The diminutive number appears above the symbol for the element and the gauge atomic mass appears below it.
The organization of the periodic table allows the elements to be grouped according to their chemic backdrop. Within the principal group elements ( Groups one-two, 13-18), in that location are some full general trends that we can find. The further downward a given grouping, the elements have an increased metallic character: they are expert conductors of both heat and electricity, solids at room temperature, and shiny in advent. Moving from left to right across a period, the elements have greater non-metallic graphic symbol. These elements are insulators, poor estrus conductors, and tin can exist in different phases at room temperature (breakable solid, liquid, or gas). The elements at the purlieus between the metallic elements (grey elements) and nonmetal elements (green elements) are metalloid in character (pink elements). They accept low electrical conductivity that increases with temperature. They also share properties with both the metals and the nonmetals.

The main group elements: Within the p-block at the boundary between the metallic elements (greyness elements) and nonmetal elements (green elements) there is positioned boron and silicon that are metalloid in character (pink elements), i.e., they have low electrical conductivity that increases with temperature.
Today, the periodic table continues to expand every bit heavier and heavier elements are synthesized in laboratories. These large elements are extremely unstable and, every bit such, are very hard to notice; simply their continued creation is an ongoing challenge undertaken by scientists around the earth.
Electron Shells and the Bohr Model
Niels Bohr proposed an early on model of the cantlet as a key nucleus containing protons and neutrons being orbited by electrons in shells.
Learning Objectives
Construct an atom co-ordinate to the Bohr model
Key Takeaways
Primal Points
- In the Bohr model of the cantlet, the nucleus contains the majority of the mass of the cantlet in its protons and neutrons.
- Orbiting the positively-charged core are the negatively charged electrons, which contribute lilliputian in terms of mass, but are electrically equivalent to the protons in the nucleus.
- In most cases, electrons fill up the lower- free energy orbitals outset, followed past the next higher energy orbital until information technology is full, and and so on until all electrons have been placed.
- Atoms tend to be virtually stable with a full outer shell (one which, after the first, contains 8 electrons), leading to what is commonly called the " octet dominion ".
- The properties of an element are determined by its outermost electrons, or those in the highest energy orbital.
- Atoms that do not have full outer shells volition tend to proceeds or lose electrons, resulting in a full outer vanquish and, therefore, stability.
Central Terms
- octet dominion: A rule stating that atoms lose, gain, or share electrons in order to accept a full valence beat out of eight electrons. (Hydrogen is excluded because it can concord a maximum of 2 electrons in its valence shell. )
- electron shell: The collective states of all electrons in an atom having the same principal breakthrough number (visualized as an orbit in which the electrons movement).
Electron Shells and the Bohr Model

Orbitals in the Bohr model: The Bohr model was developed by Niels Bohr in 1913. In this model, electrons exist within principal shells. An electron normally exists in the everyman free energy shell available, which is the 1 closest to the nucleus. Energy from a photon of calorie-free can bump it upwards to a higher energy shell, but this state of affairs is unstable and the electron quickly decays back to the basis state. In the process, a photon of low-cal is released.
Equally previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from some other, and the number of electrons information technology has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each chemical element, when electrically neutral, has a number of electrons equal to its atomic number.
An early model of the atom was adult in 1913 by Danish scientist Niels Bohr (1885–1962). The Bohr model shows the atom equally a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the diverse shells. These energy levels are designated by a number and the symbol "n." For example, 1n represents the first energy level located closest to the nucleus.
Electrons fill orbit shells in a consistent order. Under standard weather, atoms make full the inner shells (closer to the nucleus) start, ofttimes resulting in a variable number of electrons in the outermost shell. The innermost crush has a maximum of two electrons, but the next two electron shells tin each have a maximum of eight electrons. This is known as the octet rule which states that, with the exception of the innermost shell, atoms are more than stable energetically when they have viii electrons in their valence shell, the outermost electron shell. Examples of some neutral atoms and their electron configurations are shown in. As shown, helium has a complete outer electron trounce, with two electrons filling its first and merely trounce. Similarly, neon has a complete outer 2n shell containing eight electrons. In contrast, chlorine and sodium take 7 and one electrons in their outer shells, respectively. Theoretically, they would exist more energetically stable if they followed the octet rule and had eight.

Bohr diagrams: Bohr diagrams betoken how many electrons fill up each main shell. Group eighteen elements (helium, neon, and argon are shown) take a full outer, or valence, shell. A full valence trounce is the well-nigh stable electron configuration. Elements in other groups have partially-filled valence shells and proceeds or lose electrons to achieve a stable electron configuration.
An atom may proceeds or lose electrons to achieve a total valence beat, the most stable electron configuration. The periodic tabular array is bundled in columns and rows based on the number of electrons and where these electrons are located, providing a tool to understand how electrons are distributed in the outer crush of an atom. As shown in, the group eighteen atoms helium (He), neon (Ne), and argon (Ar) all have filled outer electron shells, making it unnecessary for them to gain or lose electrons to attain stability; they are highly stable as single atoms. Their non-reactivity has resulted in their being named the inert gases (or noble gases). In comparison, the group ane elements, including hydrogen (H), lithium (Li), and sodium (Na), all have one electron in their outermost shells. This means that they can reach a stable configuration and a filled outer crush by donating or losing an electron. As a consequence of losing a negatively-charged electron, they become positively-charged ions. When an cantlet loses an electron to become a positively-charged ion, this is indicated by a plus sign after the element symbol; for example, Na+. Group 17 elements, including fluorine and chlorine, have 7 electrons in their outermost shells; they tend to fill this trounce by gaining an electron from other atoms, making them negatively-charged ions. When an atom gains an electron to get a negatively-charged ion this is indicated past a minus sign after the element symbol; for example, F-. Thus, the columns of the periodic table represent the potential shared state of these elements' outer electron shells that is responsible for their similar chemical characteristics.
Electron Orbitals
Electron orbitals are three-dimensional representations of the space in which an electron is probable to be found.
Learning Objectives
Distinguish between electron orbitals in the Bohr model versus the quantum mechanical orbitals
Fundamental Takeaways
Central Points
- The Bohr model of the atom does not accurately reverberate how electrons are spatially distributed around the nucleus as they do not circle the nucleus like the earth orbits the lord's day.
- The electron orbitals are the result of mathematical equations from quantum mechanics known equally wave functions and can predict within a certain level of probability where an electron might be at any given fourth dimension.
- The number and type of orbitals increases with increasing atomic number, filling in various electron shells.
- The area where an electron is nigh probable to be institute is chosen its orbital.
Key Terms
- electron shell: The commonage states of all electrons in an atom having the same master quantum number (visualized as an orbit in which the electrons movement).
- orbital: A specification of the free energy and probability density of an electron at any point in an atom or molecule.
Although useful to explain the reactivity and chemical bonding of certain elements, the Bohr model of the atom does non accurately reflect how electrons are spatially distributed surrounding the nucleus. They practise not circle the nucleus like the globe orbits the sun, but are rather found in electron orbitals. These relatively complex shapes result from the fact that electrons acquit not just like particles, but also like waves. Mathematical equations from quantum mechanics known as wave functions can predict within a certain level of probability where an electron might exist at any given time. The area where an electron is most likely to be constitute is called its orbital.
First Electron Vanquish
The closest orbital to the nucleus, called the 1s orbital, can concord upwards to two electrons. This orbital is equivalent to the innermost electron shell of the Bohr model of the cantlet. It is called the 1s orbital because information technology is spherical around the nucleus. The 1s orbital is e'er filled before whatsoever other orbital. Hydrogen has ane electron; therefore, information technology has only ane spot within the 1s orbital occupied. This is designated as 1si, where the superscripted i refers to the i electron within the 1s orbital. Helium has ii electrons; therefore, it can completely make full the 1s orbital with its two electrons. This is designated every bit 1s2, referring to the ii electrons of helium in the 1s orbital. On the periodic table, hydrogen and helium are the simply two elements in the first row (period); this is because they are the sole elements to have electrons only in their first shell, the 1s orbital.
Second Electron Beat out
The second electron shell may contain eight electrons. This beat out contains another spherical s orbital and iii "dumbbell" shaped p orbitals, each of which can concur 2 electrons. After the 1s orbital is filled, the second electron shell is filled, first filling its 2s orbital then its iii p orbitals. When filling the p orbitals, each takes a single electron; once each p orbital has an electron, a 2d may be added. Lithium (Li) contains three electrons that occupy the first and second shells. Two electrons make full the 1s orbital, and the 3rd electron then fills the 2s orbital. Its electron configuration is 1stwo2sane. Neon (Ne), on the other manus, has a total of ten electrons: 2 are in its innermost 1s orbital, and viii fill its second shell (two each in the 2s and iii p orbitals). Thus, it is an inert gas and energetically stable: it rarely forms a chemic bond with other atoms.

Diagram of the S and P orbitals: The due south subshells are shaped similar spheres. Both the 1n and 2n principal shells have an due south orbital, only the size of the sphere is larger in the 2n orbital. Each sphere is a single orbital. p subshells are made up of iii dumbbell-shaped orbitals. Principal vanquish 2n has a p subshell, but shell 1 does not.
Third Electron Shell
Larger elements have additional orbitals, making up the tertiary electron shell. Subshells d and f take more circuitous shapes and contain five and seven orbitals, respectively. Main shell 3n has southward, p, and d subshells and can hold 18 electrons. Master shell 4n has s, p, d, and f orbitals and can hold 32 electrons. Moving abroad from the nucleus, the number of electrons and orbitals plant in the free energy levels increases. Progressing from one atom to the next in the periodic table, the electron structure can be worked out past fitting an extra electron into the next available orbital. While the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom because the orbital model specifies the different shapes and special orientations of all the places that electrons may occupy.
Chemical Reactions and Molecules
Chemical reactions occur when 2 or more atoms bond together to class molecules or when bonded atoms are broken apart.
Learning Objectives
Describe the properties of chemical reactions and compounds
Key Takeaways
Cardinal Points
- Atoms form chemical bonds with other atoms thereby obtaining the electrons they need to attain a stable electron configuration.
- The substances used in the start of a chemical reaction are chosen the reactants and the substances establish at the finish of the reaction are known every bit the products.
- Some reactions are reversible and will accomplish a relative balance between reactants and products: a state called equilibrium.
- An arrow is typically drawn between the reactants and products to betoken the management of the chemic reaction.
Key Terms
- reactant: Any of the participants present at the get-go of a chemic reaction.
- molecule: The smallest particle of a specific compound that retains the chemical properties of that compound; two or more atoms held together past chemical bonds.
- reaction: A transformation in which one or more substances is converted into some other by combination or decomposition
Chemic Reactions and Molecules
According to the octet dominion, elements are about stable when their outermost shell is filled with electrons. This is because it is energetically favorable for atoms to be in that configuration. However, since not all elements have enough electrons to fill up their outermost shells, atoms form chemical bonds with other atoms, which helps them obtain the electrons they demand to attain a stable electron configuration. When 2 or more atoms chemically bond with each other, the resultant chemical construction is a molecule. The familiar h2o molecule, H2O, consists of ii hydrogen atoms and ane oxygen atom, which bond together to form h2o. Atoms can class molecules by donating, accepting, or sharing electrons to fill their outer shells.

Atoms bond to form molecules: Two or more than atoms may bond with each other to form a molecule. When 2 hydrogens and an oxygen share electrons via covalent bonds, a water molecule is formed.
Chemical reactions occur when two or more atoms bond together to course molecules or when bonded atoms are broken apart. The substances used in the beginning of a chemical reaction are called the reactants (commonly found on the left side of a chemical equation), and the substances institute at the end of the reaction are known as the products (ordinarily establish on the right side of a chemical equation). An arrow is typically drawn between the reactants and products to signal the management of the chemical reaction. For the cosmos of the water molecule shown above, the chemic equation would exist:
[latex]2\text{H}_2+\text{O}_2\rightarrow2\text{H}_2\text{O}[/latex]
An case of a elementary chemical reaction is the breaking downward of hydrogen peroxide molecules, each of which consists of two hydrogen atoms bonded to 2 oxygen atoms (H2Oii). The reactant hydrogen peroxide is broken downwardly into water (H2O), and oxygen, which consists of two bonded oxygen atoms (Oii). In the equation below, the reaction includes 2 hydrogen peroxide molecules and two water molecules. This is an case of a balanced chemical equation, wherein the number of atoms of each element is the same on each side of the equation. According to the law of conservation of matter, the number of atoms before and later a chemical reaction should be equal, such that no atoms are, under normal circumstances, created or destroyed.
[latex]2\text{H}_2\text{O}_2 \rightarrow 2\text{H}_2\text{O} + \text{O}_2[/latex]
Even though all of the reactants and products of this reaction are molecules (each atom remains bonded to at least i other atom), in this reaction only hydrogen peroxide and water are representative of a subclass of molecules known as compounds: they contain atoms of more 1 blazon of chemical element. Molecular oxygen, on the other hand, consists of two doubly bonded oxygen atoms and is non classified equally a compound just as an element.
Irreversible and Reversible Reactions
Some chemical reactions, such as the one shown to a higher place, can go along in one management until the reactants are all used up. The equations that describe these reactions contain a unidirectional arrow and are irreversible. Reversible reactions are those that can become in either management. In reversible reactions, reactants are turned into products, but when the concentration of product goes across a certain threshold, some of these products volition be converted back into reactants; at this signal, the designations of products and reactants are reversed. This back and forth continues until a certain relative balance betwixt reactants and products occurs: a state called equilibrium. These situations of reversible reactions are often denoted by a chemical equation with a double headed arrow pointing towards both the reactants and products.
For example, in homo blood, excess hydrogen ions (H+) demark to bicarbonate ions (HCO3 –) forming an equilibrium state with carbonic acrid (H2CO3). If carbonic acid were added to this system, some of it would be converted to bicarbonate and hydrogen ions.
In biological reactions, however, equilibrium is rarely obtained because the concentrations of the reactants or products or both are constantly changing, oftentimes with a product of one reaction being a reactant for another. To return to the case of backlog hydrogen ions in the claret, the formation of carbonic acid will be the major management of the reaction. However, the carbonic acid tin likewise leave the body as carbon dioxide gas (via exhalation) instead of being converted back to bicarbonate ion, thus driving the reaction to the right past the chemical police known as law of mass action. These reactions are important for maintaining the homeostasis of our blood.
Ions and Ionic Bonds
Ionic bonds are attractions betwixt oppositely charged atoms or groups of atoms where electrons are donated and accepted.
Learning Objectives
Predict whether a given element will more likely grade a cation or an anion
Key Takeaways
Key Points
- Ions form from elements when they gain or lose an electron causing the number of protons to exist unequal to the number of electrons, resulting in a net charge.
- If there are more electrons than protons (from an element gaining one or more electrons), the ion is negatively charged and called an anion.
- If there are more protons than electrons (via loss of electrons), the ion is positively charged and is called a cation.
- Ionic bonds result from the interaction between a positively charged cation and a negatively charged anion.
Primal Terms
- ion: An atom, or group of atoms, begetting an electrical accuse, such as the sodium and chlorine atoms in a salt solution.
- ionic bond: A potent chemical bond acquired by the electrostatic attraction between 2 oppositely charged ions.
Ions and Ionic Bonds
Some atoms are more stable when they gain or lose an electron (or possibly two) and form ions. This results in a full outermost electron beat out and makes them energetically more stable. Now, because the number of electrons does not equal the number of protons, each ion has a net accuse. Cations are positive ions that are formed by losing electrons (as the number of protons is now greater than the number of electrons). Negative ions are formed by gaining electrons and are called anions (wherein there are more electrons than protons in a molecule ). Anions are designated by their elemental proper name beingness altered to stop in "-ide". For example, the anion of chlorine is chosen chloride, and the anion of sulfur is chosen sulfide.
This movement of electrons from one chemical element to another is referred to as electron transfer. Equally illustrated, sodium (Na) only has i electron in its outer electron beat. It takes less energy for sodium to donate that one electron than it does to accept seven more electrons to fill up the outer shell. When sodium loses an electron, it will take 11 protons, 11 neutrons, and only 10 electrons. This leaves it with an overall charge of +1 since there are now more protons than electrons. Information technology is at present referred to as a sodium ion. Chlorine (Cl) in its lowest free energy state (chosen the ground state) has seven electrons in its outer beat out. Again, information technology is more energy efficient for chlorine to gain one electron than to lose seven. Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons. This gives it a net charge of -i since in that location are now more than electrons than protons. Information technology is at present referred to every bit a chloride ion. In this example, sodium will donate its one electron to empty its shell, and chlorine volition accept that electron to fill its shell. Both ions now satisfy the octet rule and accept consummate outer shells. These transactions can normally only accept place simultaneously; in order for a sodium atom to lose an electron, it must exist in the presence of a suitable recipient like a chlorine atom.

Electron Transfer Betwixt Na and Cl: In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to reach an octet. In this example, sodium loses one electron to empty its trounce and chlorine accepts that electron to fill up its shell.
Ionic bonds are formed between ions with opposite charges. For instance, positively charged sodium ions and negatively charged chloride ions bail together to grade sodium chloride, or table salt, a crystalline molecule with zero internet charge. The attractive strength belongings the 2 atoms together is called the electromagnetic force and is responsible for the attraction between oppositely charged ions.
Certain salts are referred to in physiology as electrolytes (including sodium, potassium, and calcium). Electrolytes are ions necessary for nerve impulse conduction, muscle contractions, and water balance. Many sports drinks and dietary supplements provide these ions to replace those lost from the body via sweating during exercise.
Covalent Bonds and Other Bonds and Interactions
Covalent bonds result from a sharing of electrons betwixt two atoms and hold most biomolecules together.
Learning Objectives
Compare the relative forcefulness of different types of bonding interactions
Key Takeaways
Key Points
- A polar covalent bond arises when 2 atoms of different electronegativity share two electrons unequally.
- A non-polar covalent bond is one in which the electrons are shared equally between 2 atoms.
- Hydrogen bonds and Van Der Waals are responsible for the folding of proteins, the binding of ligands to proteins, and many other processes betwixt molecules.
Key Terms
- hydrogen bond: A weak bail in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or oxygen) in the same or different molecule.
- covalent bond: A type of chemic bail where 2 atoms are connected to each other past the sharing of two or more electrons.
- dipole: Whatsoever object (such equally a magnet, polar molecule or antenna), that is oppositely charged at two points (or poles).
Covalent Bonds and Other Bonds and Interactions
The octet rule can be satisfied by the sharing of electrons betwixt atoms to form covalent bonds. These bonds are stronger and much more common than are ionic bonds in the molecules of living organisms. Covalent bonds are unremarkably found in carbon-based organic molecules, such as Deoxyribonucleic acid and proteins. Covalent bonds are too found in inorganic molecules such as H2O, COii, and Otwo. One, two, or three pairs of electrons may exist shared between two atoms, making unmarried, double, and triple bonds, respectively. The more covalent bonds betwixt 2 atoms, the stronger their connectedness. Thus, triple bonds are the strongest.
The strength of different levels of covalent bonding is ane of the main reasons living organisms take a difficult time in acquiring nitrogen for use in constructing nitrogenous molecules, fifty-fifty though molecular nitrogen, N2, is the most abundant gas in the atmosphere. Molecular nitrogen consists of 2 nitrogen atoms triple bonded to each other. The resulting potent triple bond makes it difficult for living systems to interruption autonomously this nitrogen in guild to employ it equally constituents of biomolecules, such as proteins, DNA, and RNA.
The formation of water molecules is an example of covalent bonding. The hydrogen and oxygen atoms that combine to grade h2o molecules are jump together by covalent bonds. The electron from the hydrogen splits its time between the incomplete outer shell of the hydrogen cantlet and the incomplete outer shell of the oxygen atom. In return, the oxygen atom shares 1 of its electrons with the hydrogen atom, creating a two-electron single covalent bond. To completely fill the outer crush of oxygen, which has six electrons in its outer crush, two electrons (one from each hydrogen atom) are needed. Each hydrogen atom needs only a single electron to fill its outer trounce, hence the well-known formula HiiO. The electrons that are shared between the ii elements fill the outer shell of each, making both elements more stable.
Polar Covalent Bonds
There are 2 types of covalent bonds: polar and nonpolar. In a polar covalent bond, the electrons are unequally shared past the atoms because they are more than attracted to i nucleus than the other. The relative attraction of an atom to an electron is known as its electronegativity: atoms that are more attracted to an electron are considered to be more electronegative. Because of the diff distribution of electrons between the atoms of different elements, a slightly positive (δ+) or slightly negative (δ-) charge develops. This partial charge is known as a dipole; this is an important holding of water and accounts for many of its characteristics. The dipole in h2o occurs because oxygen has a higher electronegativity than hydrogen, which means that the shared electrons spend more time in the vicinity of the oxygen nucleus than they do about the nucleus of the hydrogen atoms.
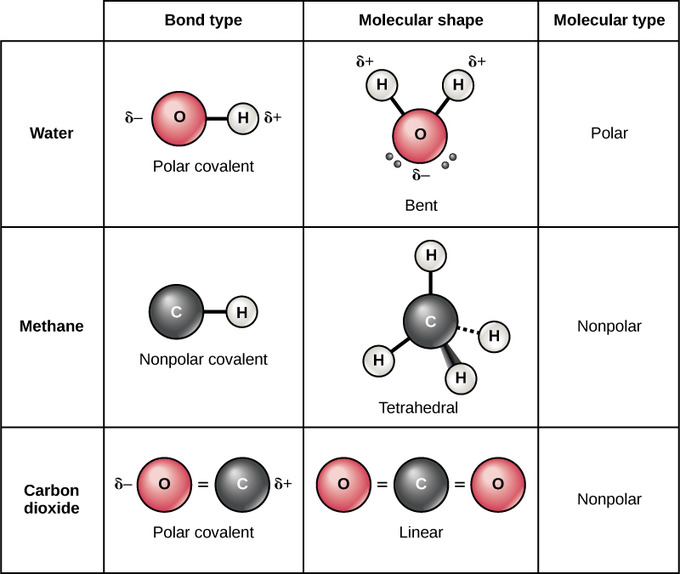
Polar and Nonpolar Covalent Bonds: Whether a molecule is polar or nonpolar depends both on bail blazon and molecular shape. Both water and carbon dioxide have polar covalent bonds, but carbon dioxide is linear, so the partial charges on the molecule cancel each other out.
Nonpolar Covalent Bonds
Nonpolar covalent bonds class betwixt two atoms of the aforementioned element or betwixt different elements that share electrons every bit. For instance, molecular oxygen (O2) is nonpolar because the electrons volition be equally distributed between the two oxygen atoms. The 4 bonds of methyl hydride are besides considered to exist nonpolar because the electronegativies of carbon and hydrogen are virtually identical.
Hydrogen Bonds and Van Der Waals Interactions
Not all bonds are ionic or covalent; weaker bonds tin can besides form between molecules. 2 types of weak bonds that oft occur are hydrogen bonds and van der Waals interactions. Without these two types of bonds, life as we know it would not be.
Hydrogen bonds provide many of the critical, life-sustaining properties of water and also stabilize the structures of proteins and Dna, the edifice block of cells. When polar covalent bonds containing hydrogen are formed, the hydrogen atom in that bond has a slightly positive charge (δ+) because the shared electrons are pulled more strongly toward the other element and abroad from the hydrogen atom. Because the hydrogen has a slightly positive charge, it'south attracted to neighboring negative charges. The weak interaction betwixt the δ+ charge of a hydrogen atom from 1 molecule and the δ- charge of a more electronegative atom is called a hydrogen bail. Individual hydrogen bonds are weak and easily cleaved; nevertheless, they occur in very large numbers in water and in organic polymers, and the additive force can be very stiff. For example, hydrogen bonds are responsible for zipping together the Deoxyribonucleic acid double helix.

Adenosine Triphosphate, ATP: Adenosine Triphosphate, or ATP, is the virtually commonly used cofactor in nature. Its biosynthesis involves the fixation of nitrogen to provide feedstocks that eventually produce the carbon-nitrogen bonds it contains.
Like hydrogen bonds, van der Waals interactions are weak interactions between molecules. Van der Waals attractions can occur between whatsoever 2 or more than molecules and are dependent on slight fluctuations of the electron densities, which can lead to slight temporary dipoles around a molecule. For these attractions to happen, the molecules need to be very close to one another. These bonds, along with hydrogen bonds, help form the three-dimensional structures of the proteins in our cells that are required for their proper function.
Hydrogen Bonding and Van der Waals Forces
Hydrogen bonds and van der Waals interactions are two types of weak bonds that are necessary to the basic building blocks of life.
Learning Objectives
Draw how hydrogen bonds and van der Waals interactions occur
Key Takeaways
Key Points
- Hydrogen bonds provide many of the critical, life-sustaining properties of water and as well stabilize the structures of proteins and DNA, the building block of cells.
- Hydrogen bonds occur in inorganic molecules, such as h2o, and organic molecules, such every bit Deoxyribonucleic acid and proteins.
- Van der Waals attractions can occur between whatever two or more than molecules and are dependent on slight fluctuations of the electron densities.
- While hydrogen bonds and van der Waals interactions are weak individually, they are strong combined in vast numbers.
Primal Terms
- van der Waals interactions: A weak force of attraction betwixt electrically neutral molecules that collide with or laissez passer very close to each other. The van der Waals forcefulness is caused by temporary attractions between electron-rich regions of one molecule and electron-poor regions of another.
- electronegativity: The tendency of an cantlet or molecule to describe electrons towards itself, form dipoles, and thus course bonds.
- hydrogen bail: The attraction between a partially positively-charged hydrogen atom fastened to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and some other nearby electronegative atom.
Ionic and covalent bonds between elements require energy to suspension. Ionic bonds are non as strong as covalent, which determines their behavior in biological systems. Even so, not all bonds are ionic or covalent bonds. Weaker bonds can as well form between molecules. Two weak bonds that occur frequently are hydrogen bonds and van der Waals interactions.

Hydrogen bonds between water molecules: The slightly negative oxygen side of the h2o molecule and the slightly positive hydrogen side of the water molecule are attracted to each other and form a hydrogen bail.
Hydrogen Bonding
Hydrogen bonds provide many of the critical, life-sustaining properties of h2o and likewise stabilize the structures of proteins and Dna, the building block of cells. When polar covalent bonds containing hydrogen form, the hydrogen in that bond has a slightly positive charge because hydrogen'southward ane electron is pulled more strongly toward the other chemical element and abroad from the hydrogen. Because the hydrogen is slightly positive, it volition be attracted to neighboring negative charges. When this happens, an interaction occurs between the δ +of the hydrogen from ane molecule and the δ– charge on the more electronegative atoms of another molecule, usually oxygen or nitrogen, or within the same molecule. This interaction is called a hydrogen bond. This type of bond is common and occurs regularly between water molecules. Individual hydrogen bonds are weak and easily broken; however, they occur in very large numbers in water and in organic polymers, creating a major strength in combination. Hydrogen bonds are too responsible for zipping together the Dna double helix.
Applications for Hydrogen Bonds
Hydrogen bonds occur in inorganic molecules, such as h2o, and organic molecules, such every bit DNA and proteins. The ii complementary strands of Deoxyribonucleic acid are held together past hydrogen bonds between complementary nucleotides (A&T, C&G). Hydrogen bonding in water contributes to its unique properties, including its loftier humid point (100 °C) and surface tension.

H2o droplets on a leaf: The hydrogen bonds formed betwixt water molecules in water aerosol are stronger than the other intermolecular forces between the water molecules and the leaf, contributing to high surface tension and singled-out water droplets.
In biology, intramolecular hydrogen bonding is partly responsible for the secondary, tertiary, and quaternary structures of proteins and nucleic acids. The hydrogen bonds help the proteins and nucleic acids form and maintain specific shapes.
Van der Waals Interactions
Like hydrogen bonds, van der Waals interactions are weak attractions or interactions between molecules. Van der Waals attractions can occur betwixt any ii or more than molecules and are dependent on slight fluctuations of the electron densities, which are not e'er symmetrical around an atom. For these attractions to happen, the molecules demand to be very close to one another. These bonds—along with ionic, covalent, and hydrogen bonds—contribute to the iii-dimensional structure of proteins that is necessary for their proper function.
Source: https://courses.lumenlearning.com/boundless-biology/chapter/atoms-isotopes-ions-and-molecules/
Posted by: titsworthonvir1943.blogspot.com


0 Response to "How To Find Protons Electrons And Neutrons Of An Isotope"
Post a Comment